REACT THERAPEUTICS
Breaking the Cancer Resistance Barrier

Benefits
- To transform the clinical paradigm of anti-cancer drugs
- Enhancing current efficacy and restoring past efficacy of anti-cancer drugs
- Expanding treatment options for physicians and patients
- Extending the lifecycle of anti-cancer drugs and antibody drug conjugate
Key words
- Cancer
- Multidrug resistance
- Inhibitors
- New Chemical Entities
Intellectual Property
- 1 patent
- 1 knowhow
Partnerships & Rewards
- 2024 French Deeptech Innovation Competition Winner
- Région Auvergne-Rhône-Alpes
- 2021 i-PhD French Deeptech PhD Competition Winner
- Fonds FEDER - Région Auvergne Rhône Alpes
Laboratory
- DPM
Institutions
- CNRS
- UGA
Linksium Continuum
- Maturation
- Incubation
- Acceleration
Results
- Incorporated startups
Context
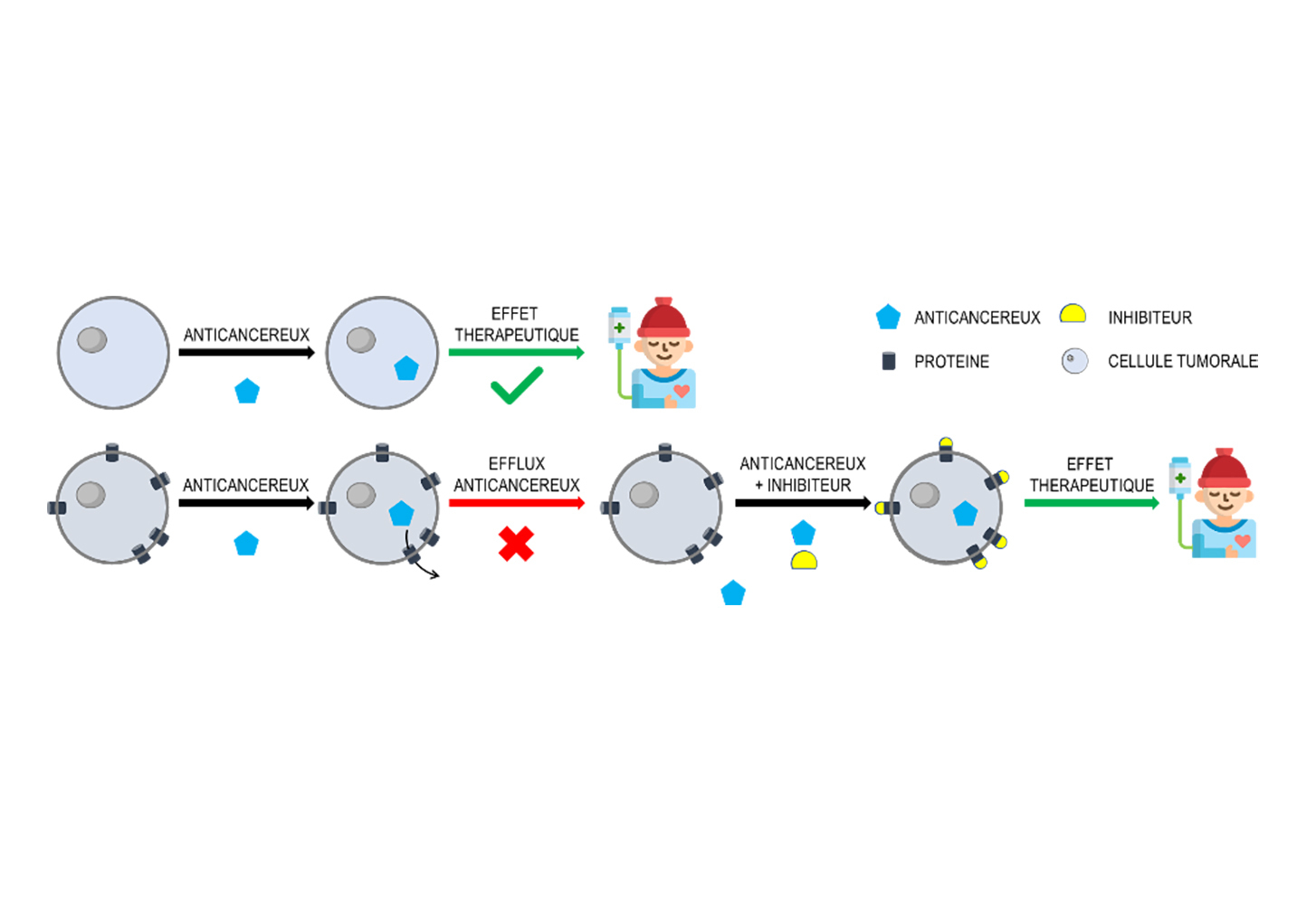
Multidrug resistance causes 90% of cancer treatment failures. We target, BCRP, which effluxes ~40 anticancer drugs —including chemotherapy, hormone therapy, and even the new antibody drug conjugates (ADCs)— impacting 14 cancer types, including 9 of the top 10 most common worldwide. Currently, no effective solution exists for inhibiting BCRP.
Technology
Our next-generation, first-in-class BCRP aims to transform the clinical use paradigm of anticancer drugs to offer patients longer, higher-quality lives. Combined with existing common BCRP-substrate anti-cancer drugs, they block efflux, restoring and boosting treatment efficacy. A very wide range of anti-cancer drugs can be ReACTivated for greater efficacy and safety by targeting BCRP inhibition.
Advantages
These BCRP inhibitors offer potent inhibition, high selectivity, no toxicity at tested doses, rapid, cost-effective synthesis, and a robust structure-activity relationship. Targeting the ADCs field presents significant growth potential in new markets. By fully harnessing the potential of its technology, ReACT Therapeutics could have a significant impact on the remission rates and quality of life of hundreds of thousands, if not millions, of patients. |
State of progress
Proof of Concept: Tumor cell studies confirm irinotecan potentiation by ValOMé, doubling efficacy and increasing mouse survival fivefold.
Toxicity: Safe in single and repeated doses (up to 75 mg/kg) in small animals, with no off-target effects across 40+ targets.
CMC: Lead compound is rapidly synthesized at scale, stable, and suitable for oral and IV administration.
Fundraising: Secured €150k (bank loans) and €465k (grants & competition). Currently raising €500k to fund R&D and IP.
Applications
ValOMé could be combined with 40 known resistant anticancer drugs used to treat 14 cancers. These drugs belong to different class of treatments: chemotherapy, targeted therapy, hormone therapy and immunotherapy.
ReACT Therapeutics is currently assessing ValOMé in colon, pancreas, and breast cancer to restore treatment efficacy.

