TERG
Antisense oligonucleotides against huntingtin for overcoming resistance of glial tumors for a better care of brain cancer patients

Benefits
- Adjuvant therapy limited to the central nervous system
- Re-sensitize for anticancer treatments
- New hope for MGMT expressing GBM
- Repositioning of existing antisense oligonucleotides
Key words
- Glioma
- Temozolomide
- Huntingtin
- Antisense oligonucleotides
Intellectual Property
- 1 patent
Laboratory
- GIN
Institutions
- CEA
- CNRS
- INSERM
- UGA
Linksium Continuum
- Maturation
- Commercialization
Results
- Available licenses
Context
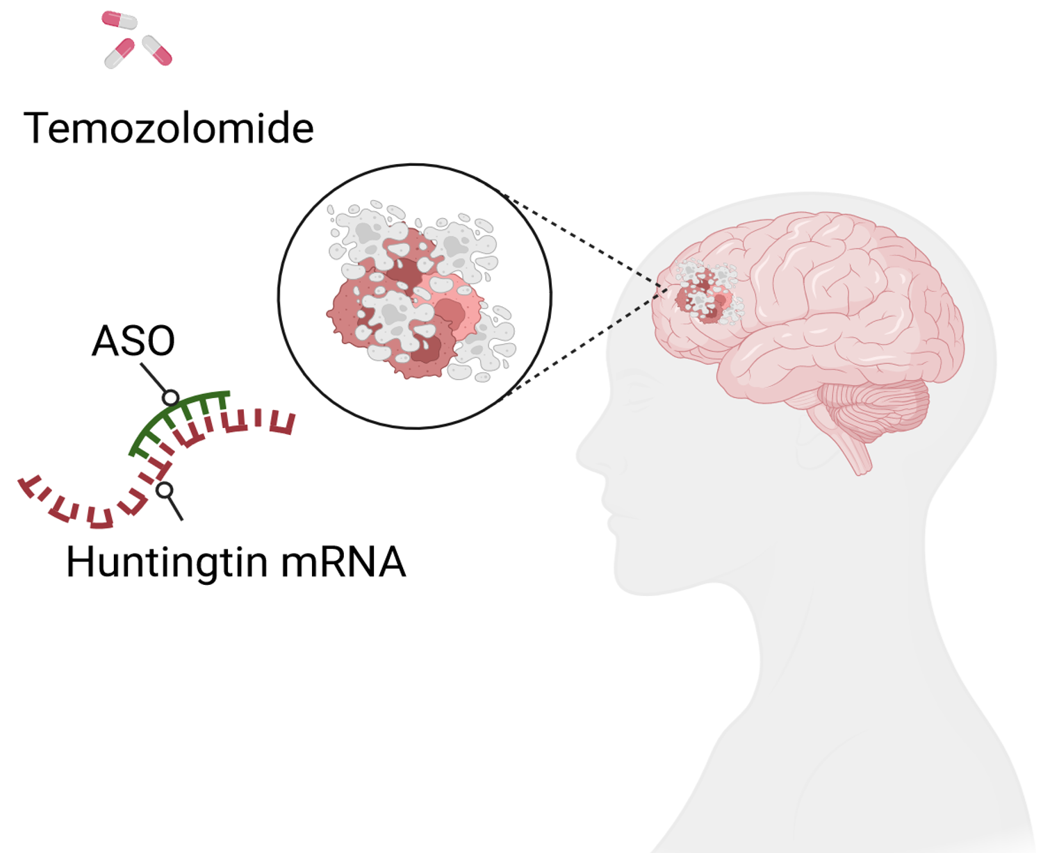
Glioblastoma multiform (GBM) is a fatal brain tumor that affects adults. The chemotherapy agent temozolomide (TMZ) is poorly efficient in resistant glioblastoma stem cells responsible for tumor growth and relapse. TERG identified a way to overcome TMZ resistance and restore the efficiency of the drug. TERG research team has demonstrated that huntingtin protein is involved in the regulation and survival of cancer cells. |
Technology
TERG proposes an innovative approach targeting the huntingtin protein in glioblastoma cells with the aim of re-sensitizing them to standard anticancer treatment (temozolomide). To achieve this, it uses antisense oligonucleotides (ASOs) with the ability to decrease the expression level of the huntingtin protein in glioma cells or glioblastoma stem cells.
Advantages
The TERG technology enhances the efficacy of temozolomide and could eventually be integrated into the current treatment protocol to improve the quality of life and survival of glioblastoma patients.
TERG offers the repositioning of antisense oligonucleotides developed to treat patients with Huntington's disease.
State of progress
In vitro tests show that huntingtin depletion by ASOs sensitizes human glioma cell lines to temozolomide by up to 70%. In vivo, ASOs administration delays tumor growth in mice treated with temozolomide and increases overall survival.
Toxicity assessments and efficacy tests on other types of gliomas are currently underway to advance the development of our solution towards clinical trials.
Applications
The use of ASOs against huntingtin as an adjuvant therapy to temozolomide may increase tumor free survival and overall survival of glioblastoma patients. The strategy presented here offers a repositioning for ASOs developed in the context of curing Huntington disease.

